| Home News and Events |

|
eOncology ReportStool-based colorectal cancer screening: simple and effective
Todd S. Crocenzi, M.D.
Medical director, Providence Gastrointestinal Cancer Program “Simplicity is the ultimate sophistication.” – Leonardo da Vinci Imagine you were asked to create an ideal cancer screening scenario. You would look for a cancer that’s detectable at an early stage, perhaps even in a premalignant stage. “Adherence rates for colorectal cancer screening are a dismal 40 to 50 percent. ”You’d also seek a cancer for which there was a good screening test or perhaps even a choice of tests, giving patients and their physicians more flexibility. Early detection and effective therapy, surgery or otherwise, would likely bring a cure. Then you’d ask for validation that such screening-led intervention reduces that cancer’s mortality rate. Forget the thought exercise — this is colorectal cancer and screening. With such an ideal scenario one might envision near 100 percent adherence. Unfortunately, adherence rates for colorectal cancer screening are a dismal 40 to 50 percent of age-appropriate adults at average risk, and they lag behind other screening programs. The first colorectal cancer screening modality with high-level evidence of support was fecal occult blood testing. Several large randomized clinical trials were published, led by a Minnesota study published in 1993 and updated in 1999.1 These important studies established guaiac-based fecal occult blood testing for colorectal cancer screening. At least in the Minnesota experience with adults over age 50, annual screening was more effective than biennial screening. Once-a-year testing became the U.S. standard. Screening advances brought colonoscopy to the guidelines, a test still viewed by many as the gold standard screening approach. But fecal occult blood testing has maintained its position in the American Cancer Society’s colorectal cancer screening guidelines since the 1980s, including in the most recent guidelines, published as a multi-society consensus document. 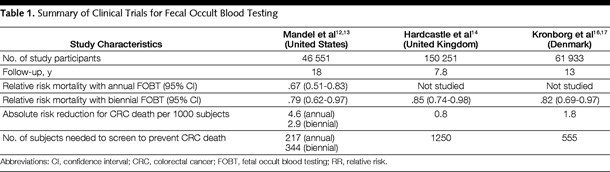 Walsh, J. M. E. et al. JAMA 2003;289:1288-1296 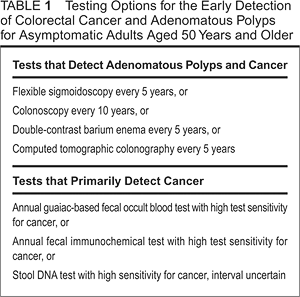
The latest American Cancer Society guidelines perhaps are an attempt to improve colorectal cancer screening by widening the net. Screening options were expanded and categorized into two options: tests that primarily detect cancers and those that detect adenomatous polyps and cancers. These recommendations provide the primary care community and oncology professionals with an array of options and guidance. Yet they also have brought uncertainties over screening intervals (stool DNA testing), reimbursement (computed topographic colonography or “virtual colonoscopy”) and availability and quality of service (colonoscopy). The U.S. Preventive Services Task Force took issue with some of the data that led to those expanded screening options. The government task force updated its own guidelines soon after the American Cancer Society publication. The task force maintained its recommendations for fecal occult blood testing, sigmoidoscopy or colonoscopy as screening options.2 Going back to basicsWhen things get complicated, an effective strategy often is to go back to the fundamentals and simplify. Fecal occult blood testing, which is included in all published colorectal screening recommendations, brings that simplification to the table. Stool testing for colorectal cancer screening includes three platforms: guaiac-based fecal occult blood testing, immunochemical occult blood testing and fecal DNA testing. The bulk of the randomized trial data supporting fecal occult blood testing, or FOBT, is primarily derived from guaiac-based FOBT technology. There has been an evolution of best practice using this technique: More clinicians now know that a single office-based rectal exam for FOBT is inadequate for screening. There’s also increased awareness that false-positive results caused by diet are less common than initially feared. And now, higher sensitivity testing (Hemoccult Sensa>>Hemoccult II) is readily available. Immunochemical occult blood testing has additional appeal in that fewer stool specimens require patient testing (typically one or two rather than three), and the test offers higher sensitivity and specificity than does guaiac-based FOBT. Further, costs of the testing, which include laboratory sample processing, have become more favorable. A recent economic analysis within the Canadian health care system showed that immunochemical occult blood testing reduced colorectal cancer deaths and lowered health care costs compared to no screening or to other accepted screening approaches, such as an annual FOBT, fecal DNA testing every three years, flexible sigmoidoscopy, CT colonography or colonoscopy.3 Fecal DNA testing may be the high-tech answer to better stool-based colorectal cancer testing, but questions remain about the frequency of testing and its affordability. This promising technology seems less prepared for primetime use when guaiac-based or immunochemical fecal occult blood tests are available. Best practice for colorectal cancer screening can be debated and should continue to evolve. Nevertheless, any effective screening is better than no screening. Patient barriers could include apprehension or discomfort over the tests, and worries about cost or inconvenience. Physician barriers could include ambivalence regarding best practice given the current range of screening options, community access to particular testing and concerns for patient and societal cost among the available test options. Stool occult blood testing can provide a “home base” to engage the patient with an effective screening method. It’s cost-effective, evidence-based and available in all clinical settings. Given the inadequate colorectal cancer screening rates and the complexities of successfully guiding patients to this end, fecal occult blood testing can offer a simple solution today. References
|
|||||||||||||||||
|
|||||||||||||||||
|
Copyright © 2024 Providence Health & Services. All rights reserved. Clinical Trials | Quality and Outcomes | News and Events | About Us | Contact Us | Make a Referral |
|||||||||||||||||