Providence Heart and Vascular Institute - eCardioVascular Beat
Case study: How to assess and treat
a patient’s genetic risk of CAD?

Doug Dawley, M.D.
Cardiology, Providence Portland Medical Center
The Oregon Clinic
I had a recent case that prompted the question posed in this headline. The patient is a 48-year-old man, previously healthy (on no meds) whose only cardiac risk factor was a family history. (His father had MI at 53, and died several years later.) He presented with a two-week history of exertional angina punctuated by an episode at rest lasting 20 minutes on the day of admission.
His initial exam was normal (BMI=24.2; BP=118/70). EKG showed T wave inversion in leads 2 and 3 and aVF. Labs revealed a troponin of 2.32, and lipid panel showed LDL=112, HDL=41, TG=128, and TC=160. “There is a genetic marker (KIF6) and a genetically determined marker (Lpa) that can be inexpensively measured to predict one’s genetic risk of CAD.”He underwent coronary angiography and was found to have a 90 percent proximal RCA lesion, which was treated successfully with a drug-eluting stent.
While genomewide association studies have identified more than 250 susceptibility loci for CAD, it has been difficult to find common abnormal loci in populations that convincingly predict CAD. Presently, however, there is a genetic marker (KIF6) and a genetically determined marker (Lpa) that can be inexpensively measured to predict one’s genetic risk of CAD, and most importantly, help in treatment decisions. I found them useful in this case.
KIF6 is a genetic test for an allele variant in the kinesin family encoded by kinesin-like protein 6. It has been associated with up to a 55 percent increased risk for CHD in five prospective studies of more than 49,000 people. The genotype for a KIF6 carrier (60 percent of the population) will have an Arg allele (e.g., Arg/Trp or Arg/Arg). A KIF6 noncarrier (40 percent of the population) will lack the Arg allele (e.g., Trp/Trp).
Taken together, the genetic association studies of CARE, WOSCOPS, WHS, ARIC, CHS and PROVE-IT-TIMI 22 suggest that the KIF6 genotype influences both the risk of coronary events and the response to statin therapy. For example, a KIF6 carrier in the WOSCOP primary prevention trial had a significant increase in risk of CHD, similar to the risk conferred by DM, high LDL or low HDL.
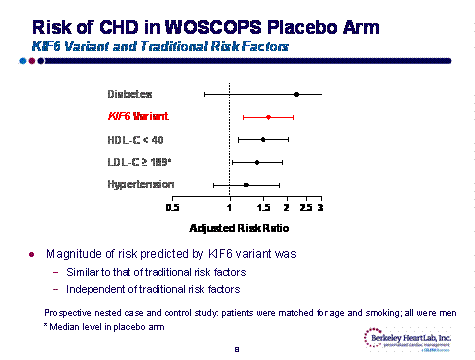
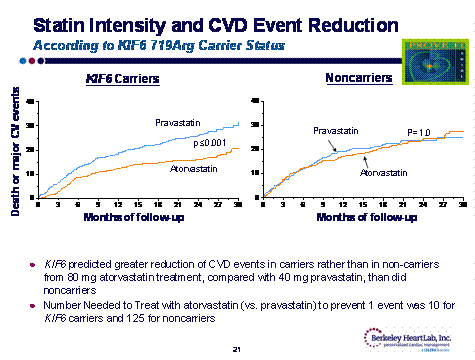
On the other hand, in WOSCOP, a KIF6 non-carrier had no increase in cardiac risk. Fortunately, the increased risk of being a KIF6 carrier can be obviated by aggressive statin therapy. In the Prove-It trial, KIF6 carriers had significantly fewer CV events when treated with atorvastatin 80 rather than pravastatin 40 mg; conversely, non-carriers received no benefit with aggressive statin therapy.
Lpa is an LDL particle with an inherited apoprotein(a) variant attached. Lpa has been linked to the promotion of both early and advanced atherosclerosis. Elevated Lpa (>30 mg/dl) is an independent risk factor for CVD, increasing risk three- to five-fold. Potential physiologic mechanisms to explain the role of Lpa in CVD involve the structural similarity of liprotein(a) to plasminogen, a fibrinolytic proenzyme.
A recent issue of New England Journal of Medicine (Dec. 24, 2009) from the Precocious Coronary Artery Disease Study (PROCARDIS), showed that two single nucleotide polymorphisms in the Lpa gene, present in 9 percent of the population, were strongly associated with both an increased Lpa level as well as risk of CVD.
Getting back to our patient, he was found to be “doubly” predisposed to CVD because he is a KIF6 carrier (Arg/Trp) and he had an elevated Lpa (72). Based on these results, I treated the patient with aggressive statin therapy (atorvastatin 80 md/d), as well as niacin (targeting a dose of 2-4 gms/d, which is the only pharmacologic intervention for Lpa). It is important to point out, though, that trials testing whether there is any outcome benefit with Lpa interventions have not been done.
In summary, KIF6 (Berkeley HeartLab: bucal swab costing $150 with no co-pay required for Medicare/Medicaid patients) and Lpa (Berkeley, VAP) are available methods to assess genetic risk for CAD. My present formula for the treatment implications of these tests is summarized:
|
Lpa >30 (BHL) |
Lpa <30 (BHL) |
| KIF6 carrier |
Aggressive statin and niacin |
Aggressive statin |
| KIF6 non-carrier |
Depending on LDL level, lower dose of generic statin and niacin |
Depending on LDL, lower dose of generic statin |
References
- Iakoubova, O., et al. “Association of the Trp719Arg Polymorphism in Kinesin-Like Protein 6 With Myocardial Infarction and Coronary Heart Disease in 2 Prospective Trials. The CARE and WOSCOPS Trials.” JACC 2008; 51 (4):435-443.
- Schiffman, D., et al. “Association of Gene Variants With Incident Myocardial Infarction in the Cardiovascular Health Study.” ATVB 2008; 28:173.
- Bare, L., et al. “Five Common Gene Variants Identify Elevated Genetic Risk for Coronary Heart Disease.” Genetics in Medicine. 2007: 9(10): 682-689.
- Schiffman, D., et al. “A Kinesin Family Member 6 Variant Is Associated With Coronary Heart Disease in the Women’s Health Study.” JACC 2008; 51 (4):444-448.
- Iakoubova, D., et al. “Polymorphism in KIF6 Gene and Benefit From Statins After Acute Coronary Syndromes
- Results From the PROVE IT-TIMI 22 Study.” JACC 2008; 51(4):449-455.
- Biomarker World Congress, Philadelphia, Pa., May 20, 2008.
- Shepherd J., et al. West of Scotland Coronary Prevention Study Group. “Prevention of Coronary Heart Disease with Pravastatin in Men with Hypercholesterolemia.” NEJM 1995; 333:1301-7.
- Clarke, Robert, et al. “Genetic Variants Associated with Lp(a) Lipoprotein Level and Coronary Disease.” NEJM 2009; 361:2518-28.
|



