| Home News and Events |

|
eOncology ReportAs liver cancer incidence rises, new systemic therapies emerge
Todd S. Crocenzi, M.D.
Medical director, Gastrointestinal Cancer Research Liver cancer is a formidable threat worldwide and is justifiably gaining attention in the United States. Much work remains to eradicate risk factors and to more broadly implement established screening strategies for high-risk individuals. Treatment for this malignancy is moving beyond the select few who are eligible for cure through resection or liver transplantation. Systemic therapy is finally emerging for this disease. Clinical trials in liver cancer are more important than ever as we build best practices for this challenging cancer threat. “U.S. oncologists will encounter more cases of liver cancer, given the linkof nonalcoholic steatohepatitis with obesity.” Liver cancer is a leading cause of cancer and cancer-related death worldwide. It is the fifth-most common cancer in the world with 554,344 cases per year.1 Although the disease is less common in the United States, there is a more recent and alarming trend of increasing incidence. This is believed to be due in part to an emerging risk factor: nonalcoholic steatohepatitis, or NASH. U.S. oncologists will encounter more cases of liver cancer, given the link of NASH with obesity, perhaps this country’s most devastating health challenge. The vast majority of liver cancer cases consist of hepatocellular carcinoma, or HCC. The malignant transformation of the hepatocyte occurs most commonly in the setting of chronic inflammation leading to fibrosis and ultimately cirrhosis. Cirrhosis represents a common transition of most causative factors involved in the development of HCC. Hepatitis B is a notable exception to the typical progression of chronic inflammation to cirrhosis to HCC. The hepatitis B viral genome can insert into the human hepatocyte genome, resulting in a “short cut” to malignant transformation without the years required for cirrhosis to develop first. Hepatitis B, hepatitis C and alcoholic liver disease represent the three dominant causes of HCC. Beyond the complexities of the causative factors, HCC remains a major challenge for the oncologic community for several reasons:
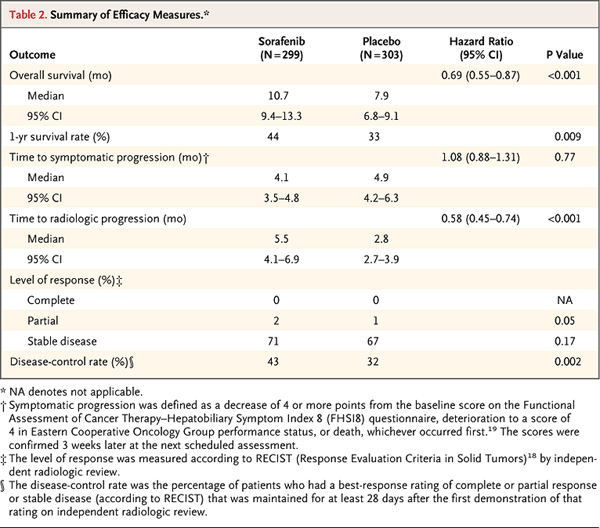
After eking out marginal benefit to our patients with HCC for several years with predominantly single-agent doxorubicin and other anthracycline use, we noted that the biologic agent sorafenib rose above the fray. Phase 2 trial data with sorafenib required forward-thinking interpretation of clinical data that included very small response rates.3 Although response rates were low, a provocative clinical benefit potential for sorafenib in HCC led to completion of the first large randomized trial showing a survival benefit of systemic therapy. The SHARP trial, presented at ASCO 2007 and published in 2008, showed a superior overall survival with sorafenib therapy (10.7 months) versus placebo (7.9 months).4
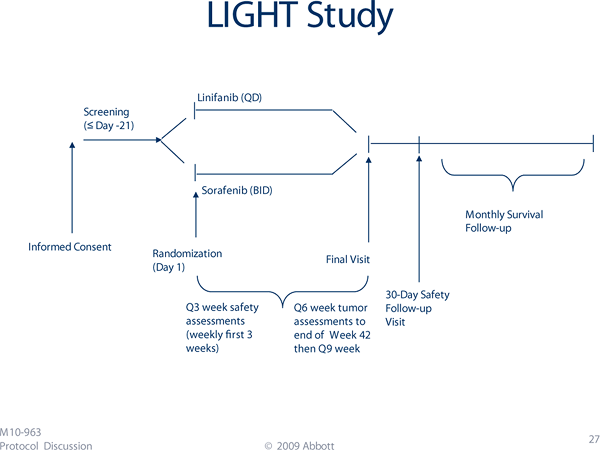
The SHARP trial focused on patients with overall good liver function (based on Child-Pugh class A), as have many of the therapies to date for this malignancy. Most patients had bilirubin below 2.0 mg/dL, albumin greater than 3.5 g/dL, INR <1.7 and the absence of significant ascites or hepatic encephalopathy. The subsequent Asia-Pacific Liver Cancer Study showed a similar benefit in a predominantly hepatitis B-driven population of HCC.5 Oncology and rock climbingIncrementally raising one’s “high point” is the basic principle for technical rock climbing known as lead climbing. Through a series of moves using holds on a rock wall, climbers will sequentially place pieces of gear as they scale the wall. The rope lifeline tied to the climber’s harness is then secured, or “clipped,” into these gear pieces as the climber moves upward. A climber can fall only as far as that piece of secured gear (the “high point”) with the help of the belayer at the bottom who secures the other end of the rope. Much of the progress in oncology could be compared to the climber’s task of scaling a rock wall. To get to the top of a climb – or to reach the pinnacle of a cancer treatment approach – one needs to make incremental moves, securing a position and then moving ever upward. Clinical trials could be considered individual moves on a climb, sometimes coming in a series of incremental moves. A multidisciplinary effort could be considered the climber’s belayer. Basic and translational research function as the climber’s gear, playing pivotal roles as the climber moves past a particularly difficult move or “crux.” Providence Cancer Center has recently opened the Abbott M10-963 study, also called the LIGHT Study, which may provide one of those incremental moves in the climb through the management of liver cancer. This is a phase 3 randomized trial comparing sorafenib with linifanib. Linifanib (ABT-869) is an oral small molecule that provides simultaneously potent and specific inhibition of the VEGFR and PDGFR families. Tolerability is expected to be acceptable given the similarities with sorafenib, which was well tolerated in large phase 3 trial experience. The study is in metastatic or unresectable HCC with participants being Child-Pugh class A and ECOG performance status 0-1 – the same population studied in the SHARP trial. The primary outcome measure is overall survival. 
The LIGHT Study is sponsored by Abbott Laboratories. It will enroll 900 patients and is projected to conclude in February 2012. If the trial is positive, another generally well-tolerated oral therapy and in a once-daily schedule will emerge for the treatment of advanced HCC. For more about the LIGHT Study, or for answers about enrollment, contact Yue-Yun To, R.N., at References
|
|||||||||||||||||
|
|||||||||||||||||
|
Copyright © 2024 Providence Health & Services. All rights reserved. Clinical Trials | Quality and Outcomes | News and Events | About Us | Contact Us |
|||||||||||||||||