| Home News and Events |

|
eNeuroNew hope for brain hemorrhage: CLEAR IVH III trial
Lisa Yanase, M.D.
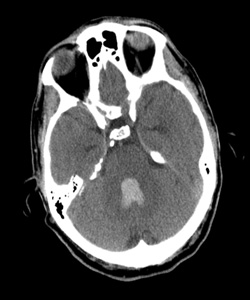
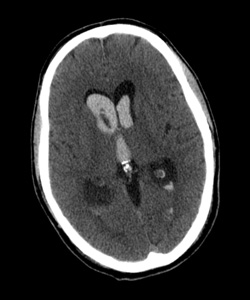
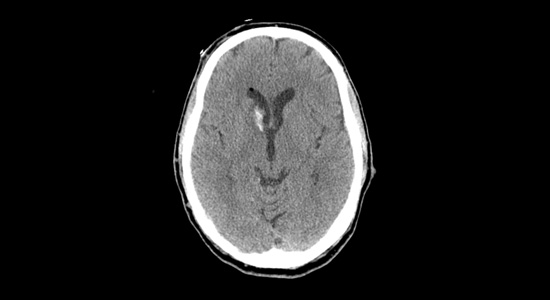
Co-medical director, Providence Stroke Center The diagnosis of intracerebral hemorrhage with intraventricular hemorrhage strikes fear into the heart of emergency physicians, and for good reason: In-hospital mortality for this condition exceeds 68 percent, even within dedicated neuro-intensive care units. Providence Stroke Center is the only site in Oregon participating in the NIH-funded CLEAR III trial (Clot Lysis Evaluating Accelerated Resolution of Intraventricular Hemorrhage, phase III), the first therapy that appears to significantly reduce mortality from this dreaded disease. “In-hospital mortality of ICH exceeds 40 percent, one-year mortality approaches 50 percent, and half of all survivors are left with disability.”The challengeIn-hospital mortality of intracerebral hemorrhage (ICH) exceeds 40 percent, one-year mortality approaches 50 percent, and half of all survivors are left with disability. Intraventricular hemorrhage (IVH) occurs in conjunction in approximately 40 percent of ICH patients, with even higher mortality of 68 to 80 percent. The added mortality and morbidity are attributable to the effects, and treatment, of acute obstructive hydrocephalus and prolonged ICU care/immobility that often lasts up to 14 days. Emergent resuscitation of these patients includes external ventricular drainage (EVD, or ventriculostomy) to relieve the intracerebral pressure. EVD drainage is continued until the IVH clears and normal CSF circulation is restored. EVD-related ventriculitis/meningitis is a potentially fatal complication of EVD placement, with risk increasing exponentially with duration of drainage. (Rates are cited from 5 to 20 percent.) In addition, the prolonged exposure to blood elements may cause scarring of the ependyma, resulting in chronic obstruction of CSF outflow and requiring shunting in up to 30 percent of survivors. The “aha” momentIn 1997, as a senior resident attending the American Neurological Association meeting, I was mesmerized by a presentation by Daniel Hanley, M.D., of Johns Hopkins University. His team had treated a series of IVH patients with intraventricular thrombolysis (both urokinase and recombinant tissue plasminogen activator had been used), with the goal of reducing the duration of EVD drainage required. Not only was there no apparent increase in morbidity/mortality, but survival was significantly above that predicted. Everyone in the room was astounded by this counterintuitive approach, so elegant in its simplicity: Get the clot out as fast as you can to shorten EVD exposure. I’ve been watching this procedure ever since, anxiously awaiting a trial to prove that it works. When the CLEAR III trial received funding, I said, “Sign us up!” The evolution of CLEAR IIIBetween 1997 and 1999, there were a handful of cohort studies showing reduced IVH mortality (20 to 31 percent versus the historical 68 to 80 percent) with intraventricular thrombolysis. In 2000, a Johns Hopkins safety trial of thrombolysis in IVH, under the FDA’s Office of Orphan Products Development, showed that intraventricular thrombolysis, most often using tPA, was safe and reduced time to clot resolution. CLEAR A, a dose-finding trial, then CLEAR B, a dose-regimen trial, have been completed, and even with low numbers, there are clear signals of efficacy. All of which leads us to CLEAR IVH III, funded by the National Institute of Neurological Disorders and Stroke. The international trial group hopes to enroll 500 patients in a placebo-controlled, double-blind study of the efficacy of intraventricular tPA. It will monitor not only safety, but neurologic and other outcomes up to a year. Genentech supplies the study drug for our consortium. Providence Stroke Center is proud to be the only stroke center in Oregon participating in this exciting clinical trial. It will be enrolling patients at Providence Portland and Providence St. Vincent medical centers under primary investigators Lisa Yanase, M.D., and David Antezana, M.D. If you have a patient with intraventricular hemorrhage, please call Providence Transfer Center at 503-216-PROV or 888-777-9599 emergently to arrange transfer to one of our participating hospitals.
|
|||||||||||||||||
|
|||||||||||||||||
|
Copyright © 2024 Providence Health & Services. All rights reserved. Clinical Trials | Quality and Outcomes | News and Events | About Us | Contact Us |
|||||||||||||||||