| Home News and Events |

|
eCardioVascular BeatPercutaneous repair of abdominal aortic aneurysm
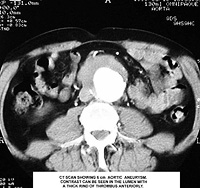
A typical contrast CT image of AAA 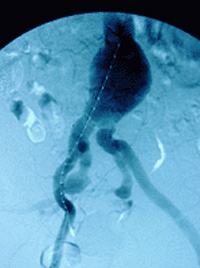
A pigtail catheter positioned both for measurement and for contrast administration 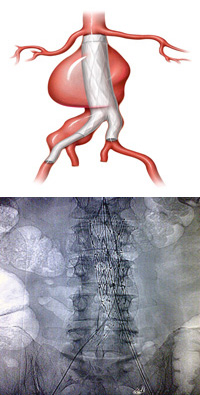
Above, an illustration and plain X-ray show a deployed aortic stent graft. 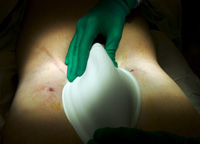
The bilateral groin punctures the patient sees after percutaneous AAA repair. In 1951, Charles Dubost1 performed the first successful repair of an abdominal aortic aneurysm, setting the stage for rapid improvement in surgical care for lethal cardiovascular problems for the rest of the 20th century. Despite patients becoming more complex, surgical morbidity and mortality results continued to improve over this period. In 1992, Juan Parodi2 reported the first deployment of an aortic stent graft in a human being. Since then, engineering improvements have led to stent grafts that are more secure and more suitable for complex anatomy. In suitable patients, aortic stent grafting for AAA has been shown to have lower acute mortality and morbidity, according to recently published data from the Medicare database.3 Currently, up to 80 percent of patients can have their aneurysm treated successfully with a stent graft. Anatomic issues and renal function are key to determining candidates for the procedure. A contrast-enhanced CT scan, using a protocol with thin, 3 mm cuts, will identify crucial anatomic features. There must be a suitable neck below the renal arteries for “seating” the stent graft; 1 cm is our current minimum for most patients. Additional factors in patient selection are size of the proximal neck (34 mm maximum), angulation of the aneurysm, size of the iliac arteries to accommodate the introducer sheath (18-24 French, depending upon the manufacturer), status of the hypogastric (internal iliac) arteries, and renal function that will allow contrast administration. The procedure is done under general anesthesia in the operating room with a hybrid surgical and endovascular team using fluoroscopic imaging. After femoral access is achieved, a guide wire is advanced up to the left subclavian artery. Intravascular ultrasound is used to confirm the sizes and measurements determined on CT scan, and the device is advanced into position and prepared for deployment. The device is then deployed through one femoral artery. The contralateral limb is deployed subsequently (bilateral femoral artery access is required to deploy an aortobiiliac graft). The goal is to seat the graft just below the renal arteries and down to the hypogastric arteries bilaterally to ensure long-term stability and to minimize late endoleak. After balloon angioplasty of the seal zones and junctions, along with a completion angiogram, the patient is awakened. At Providence Heart and Vascular Institute, this now-regular procedure is generally done by femoral artery cutdown, and is safe and effective. In some patients, it is even possible to do the entire operation percutaneously.4 After positioning Perclose devices into each femoral artery, the procedure follows the manner described above. After the sheath is removed, however, the Perclose devices are used to repair the femoral arteries. The patient is then awakened and transferred to a surgical floor. He or she may be discharged home the following morning, with only bilateral groin punctures as evidence of the procedure. At Providence Portland Medical Center, the two of us have done 30 of these percutaneous aneurysm repairs over two years. Three patients required conversion to an open femoral repair in the operating room via cutdown. There were no deaths or transfusions in this cohort of patients. From the patient’s standpoint, the minimum discomfort and quick return to normal activities is another leap forward in less-invasive repair of both abdominal and thoracic aortic aneurysm. ConclusionsIt is clear that endovascular repair of abdominal aortic aneurysm is safe and effective, particularly for the frail patient whose aortic aneurysm represents a major health threat. Percutaneous AAA repair represents another advance and another less-invasive option for selected patients with abdominal aortic aneurysm. References
|
|||||||||||||||||
|
|||||||||||||||||
|
Copyright © 2024 Providence Health & Services. All rights reserved. Clinical Trials | Quality and Outcomes | News and Events | About Us | Contact Us | Make a Referral |
|||||||||||||||||

